The urge of the reduction of gas emissions and the increasing demand of renewable-based energy systems have generated a tremendous thrust toward the development of new catalysts and the investigation of their basic principles of operation to eventually drastically improve their performances.
Electrocatalysis and photocatalysis are the keys to build the ultimate toolbox to grow a renewable energy-based technology: from the production of chemicals from waste to efficient energy conversion, to compact and affordable energy storage solutions. The first task in this direction will be to increase the reaction rate and the product selectivity in the most common fuel conversion processes, such as hydrogen evolution, oxygen evolution, oxygen reaction, and carbon dioxide reduction reactions.
This IOM research line focuses on both experimental and theoretical approaches to the understanding of the electronic state transitions (i.e. oxidation, charge transfer and intermediate species formation) during the above reactions. A deeper comprehension of the reaction mechanism will drive the design of novel catalytic materials with optimized selectivity and performance.
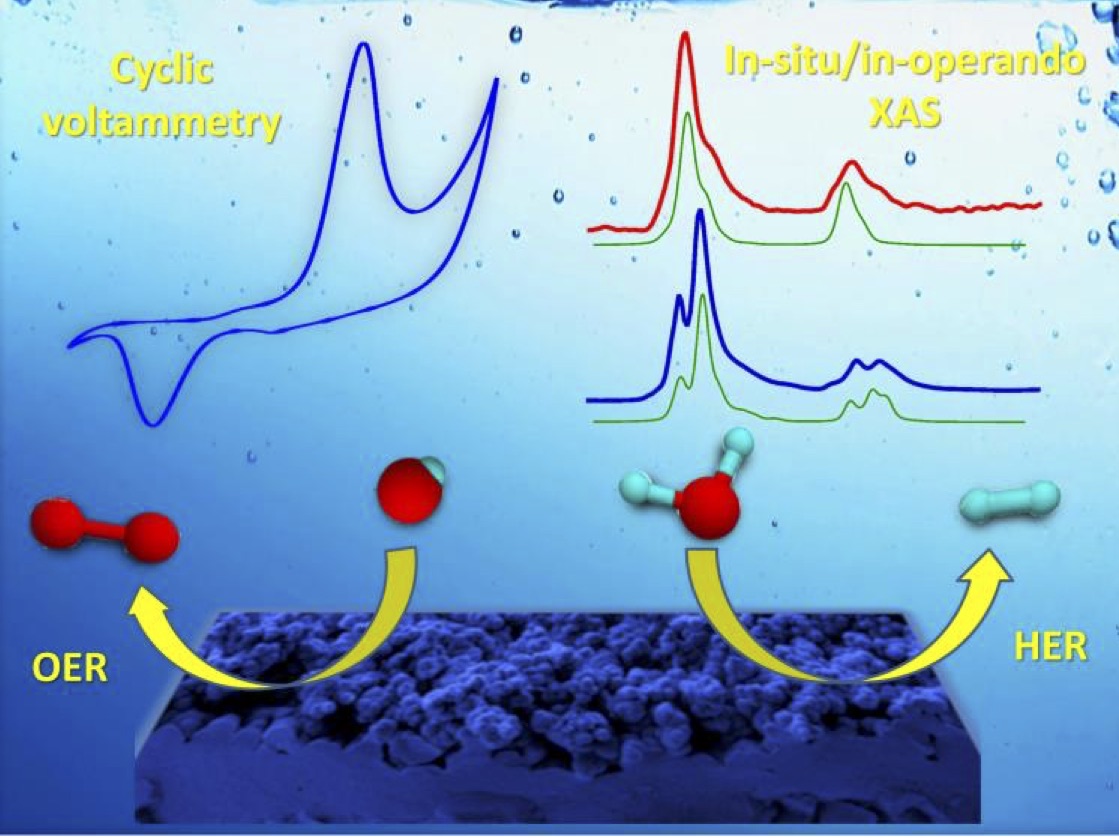
State of the art electrochemical cells are used in situ/in operando conditions together with optical and electrical excitation and X-ray or Vis-IR spectroscopy investigation to gain insights into the chemical evolution of catalytic materials under applied voltages and during charge/discharge processes.
X-ray absorption, photoemission and vibronic and vibrational spectroscopies represent powerful methods to selectively assess solid/liquid and solid/gas interface reactivity giving access to stable intermediates species, including radicals, ions, and to the interaction of electrode-electrolyte components.
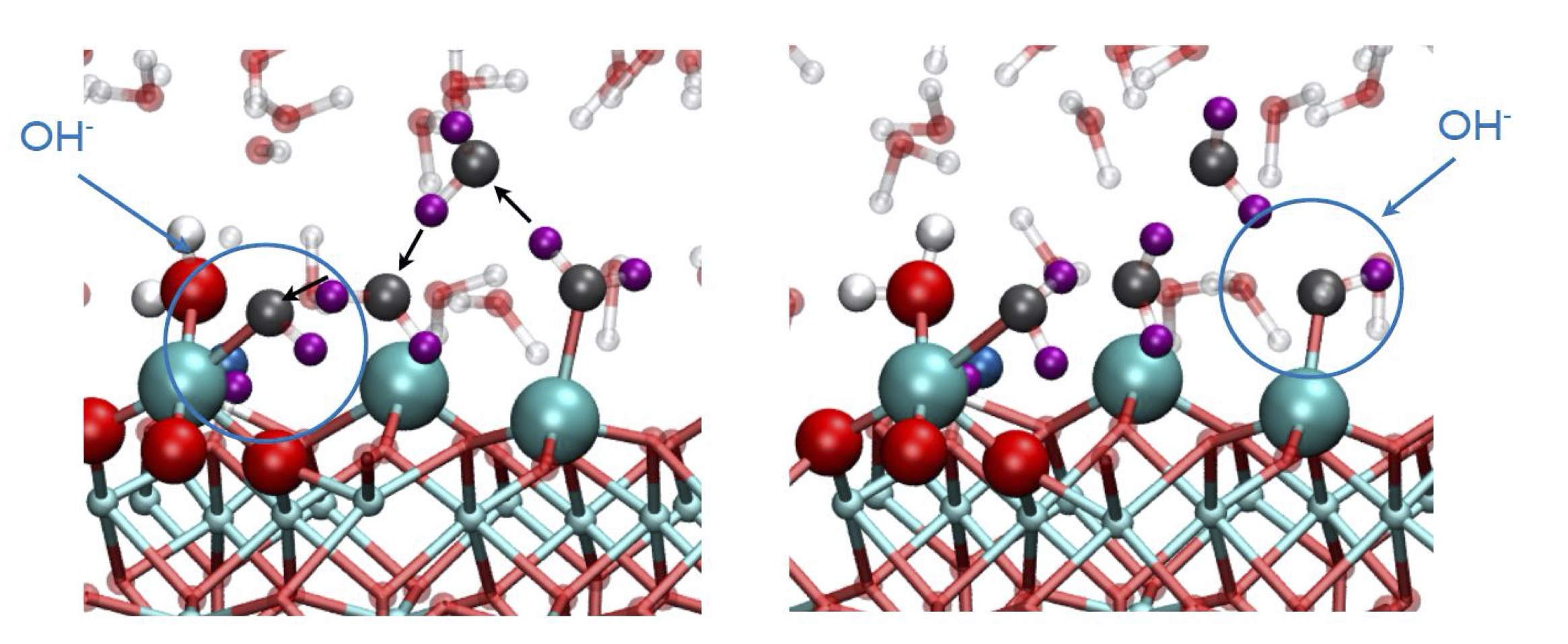
We employ computer simulations to model catalytic materials and processes at the atomic scale, using theoretical first-principles approaches based on a quantum-mechanical description of the systems of interest. We use these tools to investigate the structure of electrocatalytic surfaces and of solid/liquid interfaces, to explore mechanisms of reaction and to simulate X-ray core-level spectra. Combining theory and experiments, the goal of this research line is to gain a fundamental understanding of (photo)electrochemical processes, unraveling how the structural and electronic properties of materials influence their electrocatalytic activity.